Turning CO2 Into Chemical Gold: Affordable Nanocatalysts Could Revolutionize Climate Action

Carbon dioxide (CO2), a greenhouse gas, plays a significant role in climate change by building up in the atmosphere. To mitigate its impact, transforming CO2 into beneficial carbon products is a viable strategy. A recent study explored this approach by using nanoparticles of beta phase molybdenum carbide (β-Mo2C) as catalysts, anchored on silicon dioxide (SiO2) supports. This method accelerates the conversion of CO2 into carbon monoxide (CO), a valuable gas that can be used to produce other important compounds.
CO2 is a very stable molecule, which makes conversion of the greenhouse gas into other molecules challenging. Catalysts can be used in chemical reactions to lower the amount of energy required to form or break chemical bonds and are used in the reverse water gas shift (RWGS) reaction to convert CO2 and hydrogen gas (H2) into CO and water (H2O). Importantly, the CO gas produced by the reaction is called syngas, or synthesis gas, when combined with H2 and can be used as a carbon source to create other important compounds.
Advancements in Catalyst Technology
Traditional catalysts in the RWGS reaction are made from precious metals, including platinum (Pt), palladium (Pd), and gold (Au), limiting the cost efficiency of the reaction. Because of this, new catalyst materials and formation methods are developed to increase the practicality of the RWGS reaction as a means of lowering atmospheric CO2 and generating syngas.
In order to address the cost issues of traditional RWGS catalysts, a team of researchers from the University of Illinois in Urbana-Champaign studied the formation and catalytic activity of cheaper nanoparticle β-Mo2C catalysts on a SiO2 support to determine if the lower-cost catalyst could enhance activity levels of β-Mo2C with a silica oxide support in the RWGS reaction.
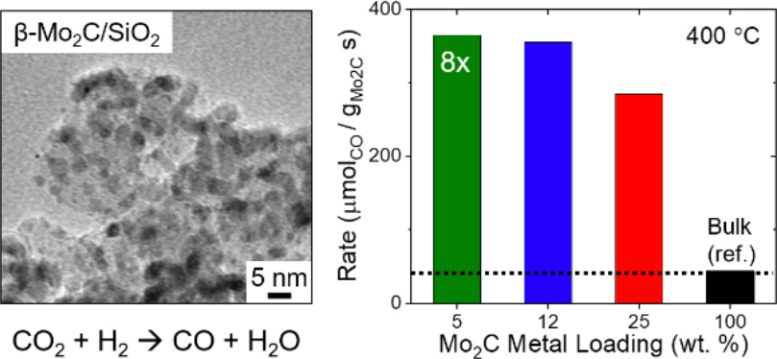
The image to the left depicts β-Mo2C nanoparticles supported on SiO2 (β-Mo2C/SiO2). The graph on the right represents the increased catalytic activity of β-Mo2C/SiO2 in CO production rate in the RWGS reaction compared to bulk β-Mo2C, represented by the black bar. Each bar represents a different percentage of Mo2C loading weight based on the mass of the SiO2 support. Catalytic activity for this data was measured at 400°C. Credit: Carbon Future, Tsinghua University Press
The team published their study in Carbon Future on April 30.
“Society is moving towards a carbon-neutral economy. Carbon dioxide is a greenhouse gas, thus any technology that can break down the carbon-oxide bond in this molecule and turn carbon into a value-added chemical could be of great interest. One important C1 chemical is carbon monoxide, which is an essential feedstock to produce a range of products, such as synthetic fuels and vitamin A,” said Hong Yang, Alkire chair professor in the Department of Chemical and Biomolecular Engineering at the University of Illinois at Urbana-Champaign and senior author of the paper.
Catalyst Structure and Effectiveness
Specifically, the researchers synthesized β-Mo2C nanoparticle catalysts absorbed onto a SiO2 support (β-Mo2C/SiO2). The amorphous structure of the SiO2 support was critical for nanoparticle formation, activity, and stability of the β-Mo2C/SiO2 catalyst. The team additionally tested cesium (Ce), magnesium (Mg), titanium (Ti), and aluminum (Al) oxides as potential supports, but the catalyst on SiO2 produced the best catalyst formation at the temperature of 650°C.
“It appears the disordered nature of amorphous silica, which behaves like glue to catalyst nanoparticles, is a key factor of our success in achieving high metal loading and the corresponding high activity,” said Siying Yu, a graduate student in the Department of Chemical and Biomolecular Engineering at the University of Illinois at Urbana-Champaign and co-author of the paper.
Importantly, the SiO2 catalyst support structure improves the catalytic activity of β-Mo2C 8-fold compared to bulk β-Mo2C. Even with improved catalytic activity, the β-Mo2C/SiO2 catalyst demonstrated high CO conversion and increased stability compared to bulk β-Mo2C in RWGS reactions.
“A major discovery of our work is a new process for producing high metal-loading catalysts made of molybdenum carbide nanoparticles. Such metal carbide catalysts are developed for converting carbon dioxide into carbon oxide at high production rate and selectivity,” said Andrew Kuhn, former graduate student in the Department of Chemical and Biomolecular Engineering at the University of Illinois at Urbana-Champaign and first author of the paper.
The researchers performed their study under reaction conditions that favored conversion to CO gas, with an H2:CO2 ratio equal to 1:1. This ratio differs from the more commonly tested ratio of less than 3:1. Reactions were also performed at temperatures between 300 to 600°C. Under these conditions, the team produced more concentrated CO, which is more efficient for downstream compound synthesis.
The team sees this research as a launching point for other catalysts that leverage support structures to increase activity. “Our ability to synthesize phase-pure metal carbide nanomaterials at high loading opens the door for the development of new catalysts for the process of CO2 utilization,” said Yang. “I hope through an in-depth study of the synthesis-structure-property relationship of this catalyst we will soon be able to uncover new important applications for value-added conversion of CO2 and the sustainable development of our economy.”
Reference: “Valorization of carbon dioxide into C1 product via reverse water gas shift reaction using oxide-supported molybdenum carbides” by Andrew N. Kuhn, Rachel C. Park, Siying Yu, Di Gao, Cheng Zhang, Yuanhui Zhang and Hong Yang, 30 April 2024, Carbon Future.
DOI: 10.26599/CF.2024.9200011
Other contributors include Rachel Park, Di Gao and Cheng Zhang from the Department of Chemical and Biomolecular Engineering at the University of Illinois at Urbana-Champaign in Urbana, Illinois; and Yuanhui Zhang from the Department of Agricultural and Biological Engineering at the University of Illinois at Urbana-Champaign.
This research was supported by the University of Illinois, Urbana-Champaign start-up fund.
相关推荐
- Game-Changing New Sensor Could Make Farming More Efficient and Groceries Cheaper
- How to Correctly Install and Maintain Pressure Transmitters
- 不使用化石燃料冶炼钢铁:太阳能打破了工业供热的1000°C壁垒
- Smelting Steel Without Fossil Fuels: Solar Power Shatters the 1,000°C Barrier for Industrial Heating
- Common Pressure Transducers Applications
- Tree Rings Reveal 2023 Was the Hottest Summer in 2,000 Years
- 将二氧化碳转化为化学黄金:经济实惠的纳米催化剂可以彻底改变气候行动
- How Does a Float Level Switch Work?
- Scientists Develop Groundbreaking Sensor That Can Wirelessly Detect Chemical Warfare Agents